
Esther Inglis-Arkell/i09.com
2 April 2015
Submariners are essentially living in an environment that should immediately kill them. In the early days of submarines, it would often find a way to do just that. One particularly horrible way involved a strange phenomenon that happened when sea water hit submarine batteries.
Electrolytes And High School Experiments
“Electrolytes” are most well-known as a marketing device. They’re what are meant to be in your sports drink, making you run faster, lift more weight, and sweat artistically. In science, they’re not just a buzzword. An electrolyte is a substance that has two qualities: It can carry an electric current through a substance, and that current can be used to split apart the components of the substance.
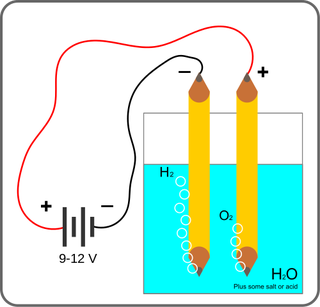
It’s not as esoteric or complicated as it sounds. You have probably done an experiment that illustrates electrolysis in high school. A professor would, via alligator clips, attach a battery to two pieces of carbon (often pencils), and dunk the pencils in water. Nothing would happen. The professor then would sprinkle salt in the water. After a while, little bubbles would appear around the pencils. The water molecules split, the oxygen going to the positively-charged pencil and the hydrogen going to the negatively charged pencil. In the process, the water particles would turn from liquid to gas.
That was electrolysis. It can be done with pure water, but since water isn’t a good conductor, that kind of electrolysis would take a lot of power. The salt is the electrolyte, allowing the current to flow and the electrolysis to proceed.
Some high school experiments go farther, asking students to try this with various possible electrolytes like baking soda and lemon juice. Which will work? Spoilers: It’s the lemon juice, because acids are good electrolytes. They help split up water, and they can split other components in a solution as well. But this became a huge problem for people on early submarines.
Electrolytes and Submarines
Submarines obviously can’t use combustion as power for the systems aboard. They had electric systems powered by batteries. These batteries contained strong sulfuric acid, and they kept a current running at all times. If some sea water leaked into the submarines – a frequent occurrence when they were still being developed and fine-tuned – first of all, most of the lights went out on the ship... which was underwater. That would be terrifying enough, but things got far worse. The batteries, still running, had set up the electrolysis lab experiment, but they wouldn’t just be splitting water. They’d be splitting salt as well. Salt is sodium chloride, and is perfectly harmless. Split it up, and“chloride” becomes “chlorine,” and “chlorine” become gaseous.

Chlorine gas was used extensively in World War I as a chemical weapon, and its effects were so terrible (and its destruction so uncontrollable) that it hasn’t been used since. It rips apart tissue, especially delicate tissue like in the lungs. When a submarine’s batteries were contaminated with sea water it either precipitated a more general malfunction or was caused by a more general malfunction. Getting to the surface, venting, or even remaining level, was difficult. So submariners were often stuck for hours in a perfectly dark, sealed metal can, that was slowly filling up with poison gas. Many died from being gassed by their own vessels.
(If you’re wondering why the electrolysis in class didn’t kill anyone chlorine gas once salt was added to the water, the experiment generated relatively little gas. If someone used enough power, enough salt, and did the thing in an enclosed room, the experiment would kill people.)


No comments:
Post a Comment